We're still moving the MLD RUSP nomination forward.
We gave/discussed this update at two NBS meetings last week, on an EveryLife Foundation Community Congress ad-hoc call, and in numerous conversations with other patient advocacy groups and bio-pharma companies. We don’t want to leave the MLD community and families out of the update loop, hence this blog post.
Status of MLD’s RUSP Nomination
Are things changing – YES! Are we panicked – NO! Do we have a plan – YES!
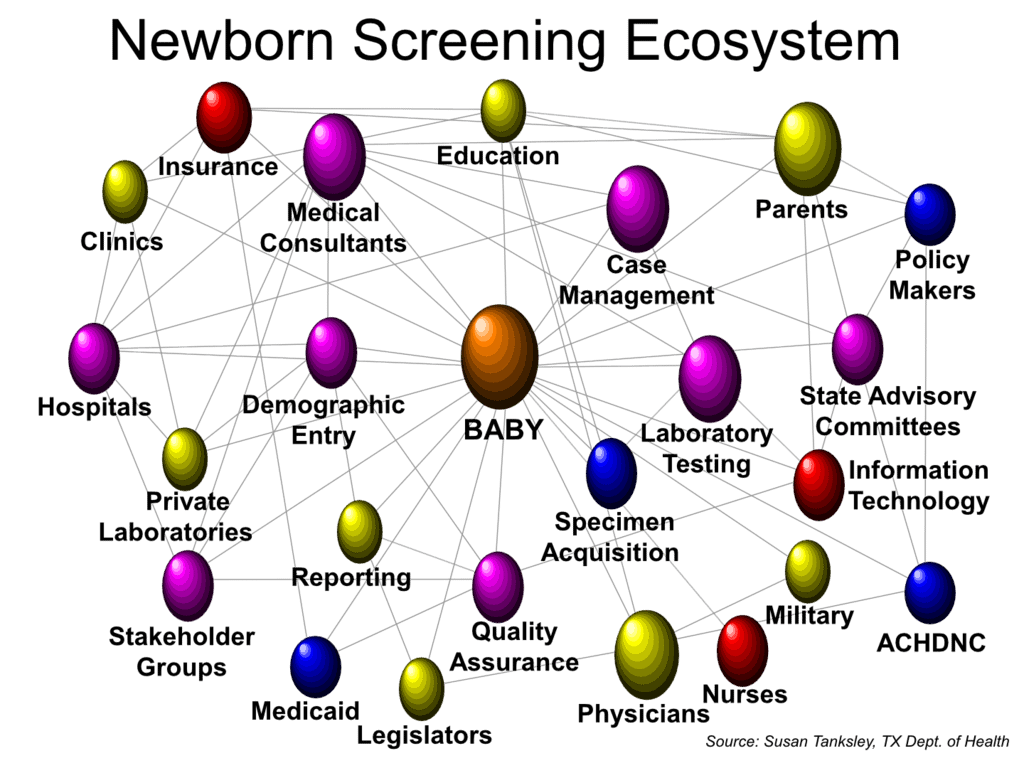
After 16 years of work and pilot studies, the MLD newborn screening assay was presented in the middle of 2024 to the ACHDNC (the Advisory Committee for Heritable Disease in Newborns and Children to the Cabinet-level Secretary of HHS (Health and Human Services)) for consideration of being added to the federal RUSP (Recommended Uniform Screening Panel). In August 2024, the ACHDNC accepted the nomination and referred it to External Evidence Review (EAR).
The EAR process has a 9-month cycle time for completion and reporting back to the Committee. The MLD Nomination has been privately described as perhaps the best nomination the EAR has seen. The Nomination was scheduled to be returned to the Committee in early May for a vote to refer to the HHS Secretary for his acceptance and formally put MLD on the RUSP.
ACHDNC Dissolved
As many of you know, the ACHDNC was terminated in early April as part of the restructuring of the HRSA division of HHS and the elimination of half a dozen other Advisory Committees. NBS was not specifically targeted, rather it was caught up in a much bigger set of changes.
No Committee – What do we do now?
We pivot and carry on. It has always been the HHS Secretary that formally put new conditions on the RUSP. With our soon to be completed External Evidence Review report, we’re actively skipping the (missing) Committee step and proceeding directly to Secretary Kennedy. We are compensating for the missing Committee review and filter by providing appropriate cover page and summary statements so the Secretary can appropriately interpret and respond to our MLD Nomination and its Expert Review.
Are Newborn Screening and the RUSP Going Away?
Absolutely not. Federal NBS resources, which used to be in the HHS department called HRSA (Health Resources and Services Administration), are now in a new department called the Administration for a Healthy America (AHA), specifically in the sub-department of Maternal and Child Health.
Real time state newborn screening is continuing. The 56 state and territory public health programs certainly benefit from federal work and support, but in the near term they will continue to screen babies just as they did 6 months ago.
The RUSP is a trusted federal resource that the states use as a trusted guide to know what to screen for next and when things are ready to proceed. We believe it is most efficient for a single in-depth federal evidence review so the states and disease advocacy do not need to replicate those review efforts 56 times. Just over 50% of the babies born in the US each year live is RUSP-aligned states (with more to come). We will not let the RUSP go away!
Do we want the ACHDNC back?
Yes and No … We want a healthy, efficient, and productive NBS advisory Committee but don’t want the “old” Committee reinstated exactly as it has been. Those of us who have been “working” with the ACHDNC for the past 15+ years have always been concerned about a variety of aspects of how the Committee operated. In fact, ten years ago, MLD Foundation launched the RUSP Roundtable to provide a forum for open and creative “not just out of the box, but as if there was no box” thinking to inspire improvements and solutions to many of these challenges.
We are actively advocating and working with other groups for the betterment of the entire rare disease ecosystem in two ways:
1) Bringing together those who want to improve a new advisory committee’s focus, operations, and efficiency to garner their insights and perspectives to collaboratively optimize a proposal for improved committee operating principles, efficiency, and scope.
2) Then, work together to advance this proposal as a win-win-win-win-win-win-win of the HHS, the legislative branch, the executive branch, the NBS ecosystem, advocacy, clinicians, pharma/biopharma, and the patients.
Do We Have the Partners and Wherewithal to Accomplish All of This?
We do. The sky may be cloudy and changing, but is not falling. MLD Foundation has been involved in federal policy since 2007, with newborn screening since 2008, with gene therapy since 2005 as participants, leaders, and respected collaborators. Never, on any of these fronts, has the path been straight or always predictable. Dean Suhr inspired the language and approach for the first and most comprehensive RUSP-Alignment legislation in California – SB 1095 in 2016. We created and have led the RUSP Roundtable since 2015, organized and led the first MLD NBS Expert Symposium that launched the MLD RUSP nomination project in 2017, followed up with a second meeting in 2020, and formally presented the Nomination to the ACHDNC in 2024. We are very well connected and trusted by rare disease and industry umbrella organizations, numerous other advocacy organizations, bio-pharma, state labs, federal HHS staff, and policy makers.
We are optimistic and must always keep advancing because the patients cannot wait, and at MLD Foundation, patients and families are why we exist.