MLD Gene Therapy
FDA Approves Lenmeldy for MLD in the USA
On March 18th, 2024, Orchard Therapeutics’ OTL-200 gene therapy was approved by the FDA under the brand name Lenmeldy™ to treat US pre-symptomatic early infantile and pre- and early-symptomatic early-juvenile patients with MLD. Lenmeldy® is the same product as Libmeldy®, the therapy’s name in Europe, which was approved by the EMA in December 2021.
The hope of Lenmeldy is to cure MLD, and based on our observations of 40+ patients in the clinical trials with over 12 years of post-therapy observation on the earliest patients, the therapy is showing extraordinary results.
Lenmeldy is an ex-vivo lentiviral-based therapy developed by the San Rafaelle Telethon Institute for Gene Therapy in Milan Italy, that was acquired by Orchard Therapeutics in April 2018 from GSK, who had licensed the technology from the San Rafaelle in the fall of 2010.
Lenmeldy is based on an autologous transplant, whereby the genetically modified blood products are harvested from the patient, genetically modified, and returned to the patient.
OTL-200 was reviewed under the FDA’s Accelerated Approval program, had both an RMAT and an Orphan Product designation.
MLD Foundation will be soon submit a nomination for MLD to be added to the RUSP, newborn screening’s federally Recommended Uniform Screening Panel in order to identify patient pre-symptomatically when the therapy is most effective. ‘s
What is Gene Therapy?
Gene therapy is based on the principle that every illness caused by a gene defect can be cured by inserting a corrected (functional) copy of the gene into the sick cells of the patient.
In the case of the MLD, it is difficult for the corrected cells to access the central and peripheral nervous system due to the Blood-Brain-Barrier which works to prevent foreign substances, including vectors carrying corrected genes, from crossing into the central nervous system (brain) and peripheral nerves.
What is ex vivo gene therapy?
The researchers at the San Rafaelle Institute in Milan, Italy, developed a gene correction and transfer mechanism that overcomes these issues to transport the functional enzyme to the affected parts of the nervous system.
Mutation: a permanent alteration in the DNA sequence that makes up a gene, such that the sequence differs from what is found in most people.
There are over 300 known mutations that can cause metachromatic leukodystrophy. MLD is an autosomal recessive disease.
Autologous: cells or tissues obtained from the same individual
The cells used in transplant traditionally come from an immune matched donor who does not have MLD. Immune matching, also known as HLA typing, assures the new cells do not reject their new host, i.e. the patient. There is always some risk of graft rejection, but this risk of Graft vs, Host Disease (GVHD) is particularly high when well-matched donors cannot be found. Finding a good match can be difficult, often takes time, and is not always possible. Interestingly, relatives are often not good HLA/donor matches.
This gene therapy uses an autologous transplant of the patient’s own repaired cells to overcome the immune response and rejection problems compared to traditional bone marrow and HSCT transplants … but remember the patient’s own cells initially carry the bad MLD genetics so they need to be modified before transplant.
Ex vivo (Latin: “out of the living”) means that which takes place outside an organism
After the patient’s own cells are harvested, the cells are purified and genetically modified ex vivo to introduce ‘healthy’ copies of the ARSA gene, and then they are returned to the patient.
HSCT – Hematopoietic Stem Cell Transplant
Clinicians and researchers have been performing cell therapy for more than 40 years, more recently using a specific cell type called a stem cell. Stem cells are capable of reproducing themselves and differentiating into many different cell types. Hematopoietic transplant means the cells are injected intravenously into the blood for their journey to the brain and peripheral nerves.
For the gene therapy the corrected cells are attached to and “piggyback” on a carrier, called a vector. As is the case in most transplant, the doctors must first “make room” for the new cells using chemotherapy conditioning.
Once the cells arrive at the brain and peripheral nerves they separate from the vector and start to reproduce and produce ARSA enzyme.
Accessing Gene Therapy
Gene therapy was approved by the EC & EMA in Q4’2020 for pre-symptomatic late infantiles and early juveniles, as well as early symptomatic juveniles. The EMA’s approval said:
“Libmeldy is indicated for the treatment of metachromatic leukodystrophy (MLD) characterized by biallelic mutations in the arysulfatase A (ARSA) gene leading to a reduction of the ARSA enzymatic activity:
• in children with late infantile or early juvenile forms, without clinical manifestations of the disease,
• in children with the early juvenile form, with early clinical manifestations of the disease, who still have the ability to walk independently and before the onset of cognitive decline.”
Accessing Gene Therapy can be a bit tricky due to the development/registration/approval phases and geographic logistics.
Currently (Sept-2021), the therapy is only available in Milano (Milan) Italy. Families and patients must relocate to Milano for several months for the gene therapy transplant. Orchard is working to open six centers in the EU to provide the therapy. In June 2021, the University if Minnesota announced they are working to open a US Pre-Approval/Compassionate Access site. (Contact info for Pre-Approval/Compassionate Access is here)
Foundazione Telethon has a program called Just Like Home that helps with practical, psychological, language and cultural support for families relocating to Italy for gene therapy. You can also apply with them for financial support.
Cost of Gene Therapy
All medical, travel, and housing expenses are generally covered when gene therapy is accessed as part of a clinical trial.
If accessed under an Early Access program the direct gene therapy expenses are covered Your insurance may cover some or all the additional medical expenses, but the rest of the travel and housing expenses are not covered.
We are here to help … Please feel free to call, email, or text us to discuss your family’s situation. We have been working with the Milano researchers and clinicians for over two decades. We are well informed about the current therapy and trial options and are happy to help you understand them so you can make an informed care decision.
Pros and Cons
- The genetic modification of the cells is fairly quick, but the transplant pre-conditioning and recovery requires several months of in-patient hospital stay.
- The transplant itself requires chemotherapy.
- The results to date show the genetically modified cells actually produce more ARSA enzyme than typical cells so they are kind of like “super producers”.
- Gene therapy is hoped to be a “one and done” therapy that gives the patient a lifetime of adequate ARSA without further therapy. Of course, the longevity of the therapy is yet to be demonstrated and proven over many decades. The first gene therapy patient was transplanted in 2010 and their first decade of results is very encouraging.
- The therapy is currently only available in taly.
Not sure what therapy or approach will help your loved one? We are happy to discuss your loved one’s situation, your desire for Pre-Approval Access, and to guide your (and your doctor’s) next steps.
We can help … Click to call, text, or email us.
Regulatory Review and Commercialization
Orchard Therapeutics acquired the rights to commercialize the gene therapy from the San Raffaele Telethon Institute for Gene Therapy (SR-Tiget, Milan, Italy), where it was initially and continues to be developed.
 Europe – EMA
Europe – EMA
Orchard’s European Union European Medicines Agency (EMA) Marketing Authorization Application (MAA) was accepted in December 2019. The MAA is being reviewed under accelerated assessment with expectations to be granted European Union marketing approval during 2H’2020.
Subsequent to EMA approval, each individual EU country also has to approve and accept the therapy based on both its medical/scientific attributes and public health viability criteria, and they must also negotiate pricing before the therapy can be accessed by citizens of a specific country.
Five countries have been identified to be the initial EU hosts of an MLD gene therapy center: France, Germany, Italy, Netherlands, and the United Kingdom. Specific facilities cities and facilities/institutions have not been identified, but it should be noted that Orchard has recently launched an MPS-IIIa gene therapy clinical trial in collaboration with Manchester University.
Orphan Designation – PTL-200 gene therapy has been granted Orphan designation by both the EMA for Europe (EU/3/10/813) and the FDA for the US.
United States – FDA
US approval for OTL-200 will come no earlier than late-2023. Requesting Pre-Approval/ Compassionate Access or potentially if another Clinical Trial opens up are the only means of US access until then. The University of Minnesota will likely be the first approved US Pre-Approval/Compassionate Access OTL-200 provider.
Orchard’s Investigative New Drug (IND) application was approved in late 2020 and opens the door to detailed discussions with the FDA about a Biologics License Application (BLA) which, once approved, will grant marketing approval in the US. In June 2021 Orchard indicated the OTL-200 BLA filing will be late 2022 or early 2023. Normally IND’s are used to gain approval for a new clinical trial, but our understanding is Orchard is not intending to launch another clinical trial, rather, they will use the IND and the existing trial data to formalize their FDA discussions to get better FDA guidance as they prepare their BLA.
OTL-200 has both a FDA Rare Pediatric Disease Designation and an RMAT – Regenerative Medicine Advanced Therapy Designation. These Designations make the program eligible for the FDA’s expedited review and approval process. The program is also eligible for a Rare Pediatric Disease Priority Review Voucher upon approval of OTL-200.
Therapy Development History
Orchard Therapeutics owns the right to commercialize the gene therapy from the San Raffaele Telethon Institute for Gene Therapy (SR-Tiget, Milan, Italy), where it was
San Raffaele Telethon Institute for Gene Therapy (SR-Tiget, Milan, Italy) was formed in 1995 as a joint venture between the Telethon Foundation and IRCCS Ospedale San Raffaele, with the mission to perform cutting-edge research in gene and cell therapy and to translate its results into therapeutic advances, focusing on genetic diseases.
The San Raffaelle team spent countless years working in the lab and then studying mice and other animals before the first in human trials started in April 2010.
Currently, all patients must travel to Milano, Italy to access this gene therapy. Typical hospital stays are 3-4 months.
Orchard Therapeutics was founded in 2015 Many members of the team were involved in some of the first research and clinical development involving ex vivo autologous gene therapy. The company went public in late 2018.
Orchard has both neurometabolic and immune deficiency programs under development. MLD is a lead disease in its neurometabolic effort.
Orchard Therapeutics acquired the intellectual property (IP) and the rights to this gene therapy from GlaxoSmithKline. GSK had previously acquired the IP in October 2010 from the San Raffaele Telethon Institute for Gene Therapy (SR-Tiget, Milan, Italy), where it was initially and continues to be developed.
MLD Foundation is Here to Help
Please feel free to call, email, or text us to discuss your family’s situation. We have been working with researchers and clinicians for over two decades. We are well informed about the current therapy and trial options and are happy to help you understand them so you can make an informed care decision.
Clinical Trials
As of May 2020, there is one gene therapy clinical trial actively recruiting in Milan, Italy.
The open trial is a Phase-III study of patients with late-juvenile MLD (up to and including 16 years old).
The Phase II cryo-study of late infantiles and early juveniles is fully enrolled. Details are retained below for your review as we monitor and discuss the outcome of the study patients. Late infantile patients should consider the Pre-Approval Access program, the link is below.
These trials are detailed below.
Phase II Cryopreserved Gene Therapy Clinical Trial
FULLY ENROLLED
As of May 2020, this late infantile/early juvenile cryo gene-therapy clinical trial has been fully enrolled and is not accepting new patients.
New LI/EJ patients can seek access under Orchard’s “Pre-Approval Access to Experimental Medicines” program (contact information at this link, too).
Please note that the separate late juvenile trial is still enrolling patients. Details here.
THERAPEUTIC GOAL
This study will assess the safety and efficacy of treatment using a cryopreserved formulation of OTL-200 in pediatric subjects with pre-symptomatic Late Infantile & Early Juvenile, and early symptomatic Early Juvenile.
An earlier study launced in 2010 is studying efficacy in pre-symptomatic late infantiles and both pre-symptomatic and early symptomatic early juveniles.
The trial sponsors hope to show that the vectors can be processed in Milan and then successfully shipped frozen to the patient in a remote facility to complete the transplant. This would enable the patient to stay closer to their home for the therapy.
Please note that this gene therapy will not repair myelin and nerve damage that has already occurred, rather, its goal is to stop further progression and damage.
THERAPEUTIC APPROACH
The therapy is an ex-vivo gene therapy using an autologous hematopoietic stem cell transplant. The vectors which transport the corrected genes will be frozen and held for a short period before being transplanted into the patient.
RECRUITING
10 patients will be recruited. The trial is an open-label single-arm study, meaning all patients receive the same therapy and there is no placebo group.
STUDY SITE
The study site is the San Raffaelle Institute in Milano, Italy.
PARTICIPATING IN THE TRIAL
All formal trial inquiries will need to be made by your doctor directly to the Principal Investigator – we can help with that process, too.
It should also be noted that this is a research project. Patients will need to participate in ongoing follow-up assessments.
Please feel free to contact us for up to date information on the trial, to discuss your loved one’s progression and its impact on trial eligibiity, guide expectations, and for assistance contacting the investigative staff to be considered for this Clinical Trial. MLD Foundation has been working with Orchard since 2017, the San Raffaelle researchers since 1995, and can quickly & efficiently guide you.
FINANCES
The cost of the therapy and all study-related testing will be paid for by the trial sponsor. Families should discuss the need for additional travel support, if any, at the time of assessment and enrollment.
STUDY GROUPS & INCLUSION
This is a research study, and like every clinical trial, has specific inclusion and exclusion criteria. We highlight the key criteria below and can direct you to the rest of the requirements as needed.
The form of the disease is determined by an assessment of the ARSA genotype and age of MLD symptom onset in their older sibling (see below), or the expected onset if there is no older sibling with MLD. Late infantile (LI) generally has symptom onset ≤ 30 months with early juvenile (EJ) symptom onset from 30 months to 6 years. (LI)
The key inclusion criteria are:
- A confirmed MLD diagnosis
- Patients must be ≤ 6 years old and have either
- an older sibling whose MLD symptom onset was ≤ 6 years old
- if diagnosed incidentally or via newborn screening (no older sibling with MLD) then eligibility will be determined after discussion with the Orchard medical monitor.
The key exclusion criteria
- If Late Infantile, either of the following is an exclusion:
- Delay in independent standing or walking combined with an abnormal neurological examination.
- Documented neurological signs or symptoms associated with cognitive, motor or behavioral impairment or regression.
- If Early Juvenile, either of the following is an exclusion:
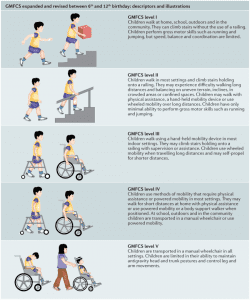
- signs or symptoms resulting in a reduction of capacity to walk independently as defined by a GMFC-MLD level ≥ 2.
- symptoms of cognitive impairment associated with an IQ < 85.
- Previous stem cell transplant, gene therapy, enrollment in other interventional clinical trials
- If Late Infantile, either of the following is an exclusion:
* Please contact us to discuss how your child may or may not fit these inclusion and exclusion criteria.
ADDITIONAL DETAILS
Primary End Points – Change in Gross Motor Function Measure (GMFM) score.
Investigators will also periodically assess change in Gross Motor Function Classification (GMFC-MLD), neurology, Nerve Conduction Velocity (NCV), brain MRI, IQ, Vector Copy Number (VCN) levels, ARSA activity level, safety, tolerability, and more.
Trial Time Frame – Recruiting started in January 2018 and should complete in 1H’2020. Patients will be followed for 8 years post-transplant.
Trial Location – Currently, all patients must travel to Milano (Milan), Italy to access this gene therapy. Typical hospital stays are 3-4 months.
Prior Trials – The first-in-human clinical trial of what is now called ORT-200 started in April 2010. There is extensive study data on the 20 patients from this first Phase I/II Clinical Trial. These patients were among the world’s first to receive gene therapy so the follow-up data is among the longest in the field gene therapy.
Access for Adults – The strategy to study in late infantile and juveniles will more quickly validate and create a pathway to treating the adult form of the disease.
ClinicalTrials.gov – Trial ID NCT03392987
Phase III Late Juvenile Trial
THERAPEUTIC GOAL
The aim of this clinical study is to assess the pharmacodynamic effect and long-term clinical efficacy and safety of OTL-200 therapy in Late Juvenile MLD patients.
An earlier clinical trial is studying efficacy in pre-symptomatic late infantiles and both pre-symptomatic and early symptomatic early juveniles.
The hope is that this single therapy will halt the progression of the disease in this new group of patients and allow the patient to live a normal life.
Please note this gene therapy will not repair myelin and nerve damage that has already occurred, rather, its goal is to stop further progression and damage.
THERAPEUTIC APPROACH
The therapy is an ex-vivo gene therapy using an autologous hematopoietic stem cell transplant.
RECRUITING
6 patients will be recruited. The trial is an open-label single-arm study, meaning all patients receive the same therapy and there is no placebo group.
STUDY SITE
The study site is the San Raffaelle Institute in Milan, Italy.
FINANCES
The cost of the therapy and all study-related testing will be paid for by the trial sponsor. Families should discuss the need for additional travel support, if any, at the time of assessment and enrollment.
STUDY GROUPS & INCLUSION
This is a research study, and like every clinical trial, has specific inclusion and exclusion criteria. We highlight the key criteria below and can direct you to the rest of the requirements as needed.
The key inclusion criteria are:
- A confirmed MLD diagnosis
- A genotype associated with the late juvenile form of MLD.
- Patient must be ≤ 16 years-old
- If symptomatic
- symptom onset ≥ 7 years-old
- If pre-symptomatic,
- must have a sibling with a late juvenile diagnosis who showed symptom onset ≥ 7 and ≤ 16 years of age
- If the patient is < 7 years old
- must have had normal motor milestone achievement
- normal neurological examination
- GMFC-MLD score of 0 (see below)
- If the patient is 7 or older
- A GMFC-MLD score of 0 or 1, i.e. able to walk independently (see below)
- IQ ≥ 85, i.e. normal cognitive function
The key exclusion criteria are previous gene therapy or current enrollment in another interventional trial.
* Please contact us to discuss how your child may or may not fit these inclusion and exclusion criteria.
PARTICIPATING IN THE TRIAL
All formal trial inquiries will need to be made by your doctor directly to the Trial Coordinator (please note: no direct patient contact is allowed). The trial coordinator will advise your doctor of the process and information to send to have your loved one considered for the trial.
Orchard Clinical Trials +44 (0) 20 3808 8286
medinfo@orchard-tx.com
Trial ID: NCT04283227
It should also be noted that this is a research project. Patients will need to participate in ongoing follow-up assessments.
Please feel free to contact us for up to date information on the trial, to discuss your loved one’s progression and its impact on trial eligibiity, guide expectations, and for assistance contacting the investigative staff to be considered for this Clinical Trial. MLD Foundation has been working with Orchard since 2017, the San Raffaelle researchers since 1995, and can quickly & efficiently guide you.
ADDITIONAL DETAILS
Primary End Points – Change from baseline in ARSA activity levels in Cerebrospinal Fluid (CSF). Change from baseline in neuronal metabolite ratio of N-acetyl-aspartate (NAA) in white matter regions of the brain – measured by MRI. Additionally, periodic assessment of Expressive Language Function Classification in Metachromatic Leukodystrophy (ELFC-MLD), Cerebrospinal Fluid (CSF) sulfatides levels, etc.
Trial Time Frame – Recruiting started in March 2020, and it will likely take well over a year to complete enrollment. Patients will be followed for 8 years post-transplant.
Trial Location – Currently, all patients must travel to Milano (Milan), Italy, to access this gene therapy. Typical hospital stays are 3-4 months. A Phase II study using cryopreservation to transport the modified vectors started in August 2018. That trial is laying the groundwork to allow this gene therapy to take place in hospitals closer to the patient’s home.
Prior Trials – The first-in-human clinical trial of what is now called ORT-200 started in April 2010. There is extensive study data on the 20 patients from this first Phase I/II Clinical Trial. These patients were among the world’s first to receive gene therapy, so the follow-up data is among the longest in the field of gene therapy.
Why is the Trial Targeted for Late Juvenile MLD? – The gene therapy has already been studied extensively in the late-infantile form of the disease because this form of the disease is the most rapidly progressing. Hence, it is easier and faster to show the efficacy of the gene therapy.
Studying efficacy in Juveniles is a logical next step to serve other parts of the MLD community.
Prior studies have already included in early juveniles, so this trial focuses on the later juvenile form of the disease.
Access for late infantiles – As of April 2020, Orchard is still recruiting for late infantiles to be part of the cryo-preservation trial. Beyond that, Orchard is in a transition from clinical trial to commercial release as they register and seeking EMA and FDA approval. If your loved one doesn’t qualify for the cryo-preservation trial, see the Pre-Approval Access (Compassionate Use) details below.
Access for Adults – The strategy to study in late infantile and then juveniles will hopefully create a pathway to treating the adult form of the disease.
Compassionate Use, Named Access, Pre-Approval Access
Compassionate Use, Named Access and Pre-Approval Access are all names for similar programs where the Food and Drug Administration (FDA) or the regulatory agency in your country allows companies to provide their experimental drugs to people outside of clinical trials.
The FDA’s criteria are:
- Your disease is serious or immediately life-threatening.
- No treatment is available or you haven’t been helped by approved treatments for your disease.
- You aren’t eligible for clinical trials of the experimental drug.
- Your doctor agrees that you have no other options and the experimental treatment may help you.
- Your doctor feels the benefit justifies the potential risks of the treatment.
- The company that makes the drug agrees to provide it to you.
Generally, companies prioritize their limited supplies of trial materials and resources, in this case, staff, product, and processing capacity, to their Clinical Trials and ongoing studies. It should also be noted that the company’s financial obligations may be reduced with these special access programs. Both your doctor and the company need to be in agreement that the desired therapy will be beneficial.
Also note that it’s unlikely you will be granted Compassionate Access if your child does not fit the EMA “indication”, i.e. what the therapy was approved for:
• in children with late infantile or early juvenile forms, without clinical manifestations of the disease,
• in children with the early juvenile form, with early clinical manifestations of the disease, who still have the ability to walk independently and before the onset of cognitive decline.”
With that said, you won’t get access if you don’t ask!
How to Access Gene Therapy Now
Orchard Therapeutics has a Pre-Approval Access to Experimental Medicines policy you can find here. Key highlights of the policy are:
- They seek to first complete enrollment in existing studies
- The potential benefit and risk of the therapy
- Exhausting other alternatives
- Availability of resources and product supply
- Orchard requires your doctor to make the inquiry on your behalf – they do not accept direct patient inquiries.
- The commit to a speedy response, currently approximately 5 days.
Please ask your doctor to contact Orchard Medical Affairs to start the process: medinfo@orchard-tx.com
Not sure what therapy or approach will help your loved one? We are happy to discuss your loved one’s situation, your desire for Pre-Approval Access, and to guide your (and your doctor’s) next steps.
We can help … Click to call, text, or email us.
WHAT IS GMFC-MLD?
Gross Motor Function Classification System or GMFCS is a 5 level clinical classification system that describes the gross motor function of people on the basis of self-initiated movement abilities. Particular emphasis in creating and maintaining the GMFCS scale rests on evaluating sitting, walking, and wheeled mobility. Distinctions between levels are based on functional abilities; the need for walkers, crutches, wheelchairs, or canes / walking sticks; and to a much lesser extent, the actual quality of movement.
The original version of the GMFCS was developed in 1997 for cerebral palsy. The current GMFC-88 has been adapted to the unique characteristics of MLD and is refereed to as GMFC-MLD.
The following three graphics may help you to preliminarily self-determine what GMFCS-MLD level your loved one might be. The 3rd graphic provides more detailed descriptions broken out by age group from birth to 18 years old.

Click on the image below to enlarge it to show more details by age group from birth through age 18:
© 2020, MLD Foundation, all rights reserved.

 Europe – EMA
Europe – EMA